Research Projects
Role of PTEN in pathological vascular remodeling:

Using an unbiased screen to identify novel genes regulating a unique smooth muscle phenotype expressed in early vascular development and during vascular disease progression, we uncovered a role for the tumor suppressor, PTEN, as an essential regulator of smooth muscle phenotype. A parallel, but separate research direction identified the extracellular heparan sulfate proteoglycan, perlecan, as an essential component driving smooth muscle differentiation and vessel maturation; subsequent studies linked perlecan regulation of PTEN to vessel maturation. Therefore, through two parallel, but independent research paths, we were the first to identify PTEN as an indispensable regulator of smooth muscle differentiation and vessel development. At this time, while PTEN was actively being studied as a tumor suppressor commonly mutated in a wide variety of human cancers, very little was known of its physiological activity in other systems and there was no information of PTEN regulating SMC function.
Our most recent original findings revealed a novel role for PTEN as a transcriptional co-factor for the transcription factor, SRF, a master smooth muscle differentiation regulator, never before been demonstrated in any cell system. Translationally, using coronary arteries micro-dissected from explanted human hearts at the time of transplant, we found that loss of PTEN is associated with atherosclerosis progression, and the extent of SMC PTEN loss correlates with lesion severity. Most recently, our findings show that systemic PTEN upregulation protects against pathological vascular remodeling making PTEN a highly significant therapeutic target. Collectively, we demonstrated that PTEN regulates cell autonomous events, including differentiation, proliferation, and pro-fibrotic vascular remodeling, as well as non-autonomous events, including inflammation. Therefore, our findings support that PTEN actively suppresses multiple factors and pathways involved in the major events driving pathological vascular remodeling and vascular disease progression.
Contribution of smooth muscle cell-derived resident stem cells in vascular homeostasis and pathological vascular remodeling:

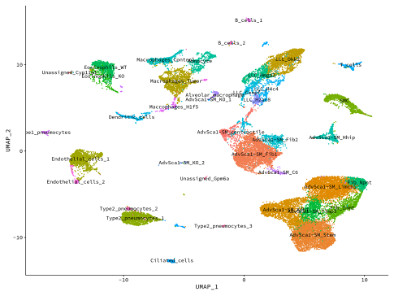
Using our smooth muscle cell fate-mapping system, we were the first group to definitively show that the vast majority of intimal smooth muscle cells in the setting of restenosis derive from resident mature smooth muscle cells. In addition, we demonstrated that distal muscularization of precapillary pulmonary arteries in the setting of pulmonary hypertension originates from mature smooth muscle cells. Our most recent novel and exciting area of research has been our discovery that mature smooth muscle cells are endogenously reprogrammed to give rise to a distinct population of resident vascular stem cells (AdvSca1-SM cells), a highly novel finding that has important implications to regenerative medicine. Our data suggest these cells play important roles in arterial homeostasis and disease and manipulating these cells in situ could improve therapeutic applications in settings such as atherosclerosis/restenosis, aneurysm formation, ischemic tissues, and tumor angiogenesis.
Our studies have shown that AdvSca1-SM cells are major contributors to pathological vascular remodeling and fibrosis, atherosclerosis progression, and organ fibrosis, including cardiac and kidney fibrosis. Current research is focused on defining mechanisms underlying smooth muscle cell reprogramming to AdvSca1-SM cells as well as their regulation during disease progression.
Role of the tumor microenvironment in lung cancer progression and metastasis:
We developed a novel lung cancer metastasis model in order to define the role of the lung tumor microenvironment on lung cancer progression, to define specific signaling pathways activated in both cancer cells and the tumor microenvironment that regulate cancer progression, and to test potential therapeutic agents. This system is an immunocompetent orthotopic mouse model, in which murine lung cancer cells derived from C57BL/6 mice are directly injected into the lungs of syngeneic mice. These cells form a primary tumor which over time will form secondary pulmonary tumors and metastasize to the liver and brain. We have used this system to study the role of eicosanoids, PPARgamma signaling, immune cell regulation, and effects of checkpoint inhibitors on lung cancer progression and metastasis.
Most recently, we have shown that smooth muscle-derived AdvSca1-SM cells are activated in response to tumor formation and differentiate toward a myofibroblast phenotype to significantly contribute to lung cancer progression and metastasis. Ongoing research is focused on fully defining their contribution to cancer progression and mechanisms leading to their activation and differentiation to myofibroblasts.
